Chemically Induced Sight Glass Failures - Stress Catalyzed Corrosion

Click to watch a video talk on this segment
The most common cause for sight window failures is chemical corrosion, which has undoubtedly ruined more sight glasses than all other mechanisms of failure combined. Chemical corrosion is nothing more than undesired chemical reaction, and will happen to any material under the right chemical environment. Thus, the only way of preventing chemical corrosion is to understand the chemical interactions between the process environment and all wetted components of the sight window.
The progress of a chemical reaction can be arrested by slowing its reaction kinetics or altering its thermodynamic equilibrium. Ideally, using window material that is thermodynamically unreactive towards the process environment would eliminate corrosion altogether. Unfortunately, transparent materials suitable for use as a window element are commercially available in relatively few varieties. Plastics and silicate glasses constitute the vast majority of usable window materials. While zinc selenide or sapphire might be used in specific applications, these crystalline materials are very expensive and priced out of most applications. Plastics are widely used, but they usually too plastic to be used in challenging conditions; their insufficient rigidity makes them unsuitable for a number of applications, and their service temperature limits tend to be low as well. As far as thermodynamic reaction potential is concerned, a plastic would be well suited for holding, say, molten sodium hydroxide. But a temperature high enough to keep sodium hydroxide molten would also degrade or destroy most plastics. So for mechanical reasons, thermodynamically compatible materials are often ruled out.
In the end, silicate glass is still the most commonly used material, even in cases where it is not thermodynamically compatible with the process fluid, which means that thermodynamic potential for corrosion is always present. The following discussion is limited to one type of common silicate glass corrosion, although the concepts are applicable to any chemical corrosion.
Hydroxyl Attack on Silicon Oxygen Bond
The most common and most studied corrosion mechanism for silicate glass is the reaction of hydroxyls with the silicon-oxygen matrix of silicate glasses. Silicate glass is made up on silicon and oxygen atoms, with different cations that affect the glass properties. Silicon atoms in a glassy matrix are not particular about the oxygen atoms to which they bond. If an oxygen atom from a hydroxyl is very close to a silicon atom on glass surface, the silicon atom can switch it bond from the oxygen in the glass matrix to the oxygen on the hydroxyl. Of course, water is full of hydroxyls, and hydroxyl from water will attack glass. This mechanism is shown below as Figure 3 (taken from Michalske TA and Frieman SW (1983). A molecular mechanism for stress corrosion in vitreous silica. Journal of the American Ceramic Society, 66: 284-288.).
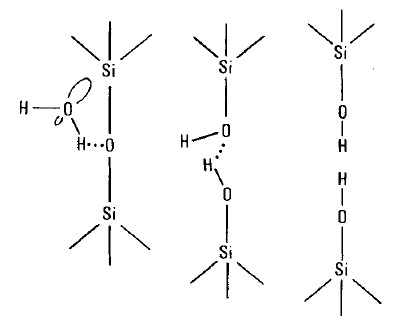
Figure 3: Water corrosion of glass.
The fact that water attacks glass comes as an unexpected surprise to most people, as everyone is used to seeing water cups made of glass. But even in that application, old glass cups exhibit a dull surface, and their original luster cannot be restored by cleaning; the water has attacked and etched the surface of the glass. At room temperature, reactivity of water towards silicate glass is negligible. Among other factors, water at room temperature does not dissociate much into proton and hydroxyl.
Presence of additional hydroxyl anions greatly accelerates the corrosion of glass. Aqueous hydroxides will attack glass at room temperature, although at slow enough rate to permit the use of glassware for laboratory experiments involving hydroxides. Since silicon in glass is inherently reactive towards the oxygen end of hydroxyl anions, the only certain method of stopping their reaction is by coating the glass so that the two chemical reactants never come together.
Arrhenius Behavior
The most important ingredient that turns normally harmless aqueous fluids corrosive is temperature. This dependence is shown below in a typically used formula.
Great many non-biological chemical reactions can be described by this formula or something that looks like it. K is the reaction rate constant, E the activation energy, and T is the absolute temperature. This formula describes the rate of corrosion of glass reasonably well. The most important part of this formula is its exponential structure. This exponential captures the fact that water is harmless to glass at 300 degrees Kelvin, room temperature, yet attacks glass voraciously at 450 degrees Kelvin.
This formula does not include a pressure term, and most liquid phase reaction rate equations do not include pressure terms, yet this is a risk related to pressure. Most sight windows designed for ANSI flange applications are rated at 150 PSIG, since that is a standard value for ANSI specifications. And water is normally harmless towards glass. But if that pressure is being used to hold water in a superheated state-a very common application-then then pressurized operation is an indication of extreme chemical aggressiveness of water. Water as process liquid, which is normally harmless towards glass, 150 PSI process pressure, and 350 degrees Fahrenheit process temperature are all within specifications of most commercially available sight windows. In reality, if these specifications are used to hold water in a superheated state, this environment will attack glass aggressively. So the fact that a given sight window's specification accommodates these conditions with large margins of safety does not mean that real risk factors have been addressed in any way at all.

Figure 4: Sight glass corroded by water. 33 expansion borosilicate glass (Corning Pyrex, Schott Borofloat, etc.).
Figure 4 shows a real life example of a sight glass that was subjected to such conditions, with superheated water as the process fluid. It should be extremely obvious that this is not a small or slow effect. This degradation occurred over a period of months, not decades. This glass had a machined step at the edge of the glass to begin with, but it is clear that several millimeters of glass was lost from all surfaces. This glass should have never been subjected to this environment.
The only resolution to this problem is to ensure that water molecules do not come into contact with the silicon atoms of the glass. Some sort of shielding is necessary to prevent this, either a coating or a shielding layer. Normally, Teflon coating should be used for applications like this. Of course, glass will corrode if the coating is scratched.
Stress Catalysis of Corrosion
Note that the exponent of the Arrhenius equation shown above includes the activation energy "E" term. Thus, along with temperature, there is another exponential dependence. Presence of catalysts alters this value, which describes the radical acceleration of reactions when suitable catalysts are present. It turns out that mechanical stress acts as such a catalyst.
Catalytic effect of mechanical stress is universal, and commonly observed. Those who were unfortunate enough to own pickup trucks made during the 1980' s undoubtedly recall the fact that those trucks started rusting at the stressed points of the frame. Glass corrosion exhibits the same behavior.
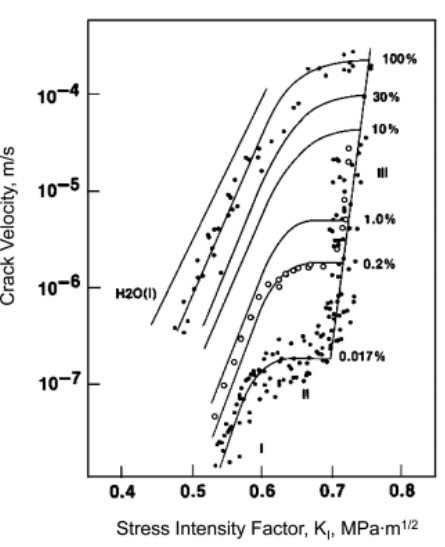
Figure 5: Influence of water vapor on crack propagation.
Figure 5 charts the quantitative effects of water on fractures (taken from Wiederhorn SM (1967). Influence of water vapor on crack propagation in soda-lime glass. Journal of the American Ceramic Society, 50: 407-414.). As expected, the vertical axis is plotted on a logarithmic scale, as appropriate for charting exponential dependence. A modest increase in stress intensity increases the crack propagation by a factor of ten.
This effect ties into the gasket seating stresses discussed around Figure 2. The volume of glass right below the gasket is the most stressed point in the glass. Thus, this location is where the corrosion occurs at the highest rate. This is the principle reason that the corroded piece of Figure 4 has virtually no side wall left; this piece was sealed along the side wall, so the sealing stress was acting upon the side wall, which then corroded away.
Note that hydroxyl attacks any kind of silicate glass, and there is no treatment of the silicate glass that can arrest this attack. Some chemical treatments might reduce the corrosion rate, but corrosion cannot be stopped. This is also true of compression strengthened glasses. Tempering glass or fusing glass into metal rings strengthens glass by inducing compressive stresses in the glass. While compressive stresses reduce the rate of hydroxyl attack somewhat, they certainly do not arrest it, and does not offer any meaningful improvement for actual service.
For fused glass, as shown in Figure 6, there is another issue. While the bulk of the stress on the glass is compressive, it is not possible to avoid all t stress components in this geometry. The glass elements of these sight windows are strengthened because the thermal expansion coefficient of metal is greater than that of glass. So when they cool down below glass annealing temperature, the metal ring contracts more than the glass and it squeezes the glass with enormous force. However, the contraction takes place along thickness dimension as well, so the metal ring wants to become thinner than the glass does. This contraction mismatch in the thickness direction causes an unavoidable, and very significant, tensile stress along the periphery of the glass. If there is any corrosive weakening of the glass, this location will start cracking.

Figure 6: Fused glass with cracks along the periphery.
Figure 6 shows such cracks. If the environment is corrosive enough, the corrosion will create a propagating fracture along this periphery. And if the fracture propagates far enough, then the shear stress between glass and metal exceed the capacity of the remaining, intact glass to resist that shear, resulting in a small shard of glass breaking away. After the break, fresh glass surface will be exposed, and that will become the most stressed portion of the glass. Thus, this interplay of stressed enhanced corrosion and shear stress between glass and metal will greatly accelerate the rate of glass loss, with most of the glass loss occurring through breaking rather than corroding. Fused glass is almost never the best choice if there is a possibility of glass corrosion taking place.
Unfortunately, there is a common notion spread by vendors that these types of sight windows are "just better." Such oversimplifications are obviously silly. This type of window represents a trade-off in which mechanical strength was enhanced at the expense of chemical durability. In situations where silicate glass is attacked by its environment, this type of silicate glass window is usually more vulnerable than equivalent, unstressed glass, and it is really best to avoid altogether placing any material with any fluid that will corrode it.
< Back to Introduction
| This web site is provided by: |  | Copyright 2015, All Rights Reserved. |



